Patent & certification
Patent & certification
Patents

- Cancer genetic analysis methods by multi-amplification double signal amplification and kits for cancer genetic analysis for them
- Detection method of target nucleic acid sequence by multi-amplification double signal amplification
- Brca gene mutation detection method by multi-amplification dual signal amplification
- Primer for nucleic acid amplification with dumbbell structure and method of detecting beneficial bacteria in the intestine using it
- Oligonucleotides in dumbbell structures and how gene variations are detected using them
- Dumbbell structure oligonucleotides, primers for boosting nucleic acids, including them, and nucleic acid amplification methods using them
- Method of detecting sex propagation disease causative agents using liquid bead arrays and multi-polymerase chain reactions
- DNA chip genotype analysis method for genotype analysis of pharyngeal virus
- Identification of tumor-caused pharyngeal virus types by double-multiple reverse-reverse-reverse polymerase chain reactions
- Method of detecting tumor-caustic pharyngeal virus using liquid bead array and multi-polymer enzyme chain reaction method
- Complex biomarkers for breast cancer diagnosis and their use
※ Trademark registration for all molecular diagnostics: DLP, MANSA, CARENOMICS

Cancer genetic analysis methods by multi-amplification double signal amplification and kits for cancer genetic analysis for them

Detection method of target nucleic acid sequence by multi-amplification double signal amplification

Brca gene mutation detection method by multi-amplification dual signal amplification

Primer for nucleic acid amplification with dumbbell structure and method of detecting beneficial bacteria in the intestine using it

Oligonucleotides in dumbbell structures and how gene variations are detected using them

Dumbbell structure oligonucleotides, primers for boosting nucleic acids, including them, and nucleic acid amplification methods using them

Method of detecting sex propagation disease causative agents using liquid bead arrays and multi-polymerase chain reactions

DNA chip genotype analysis method for genotype analysis of pharyngeal virus

Identification of tumor-caused pharyngeal virus types by double-multiple reverse-reverse-reverse polymerase chain reactions

Method of detecting tumor-caustic pharyngeal virus using liquid bead array and multi-polymer enzyme chain reaction method

Complex biomarkers for breast cancer diagnosis and their use
Certificates

- CE-IVD certification - DLP™Q COVID-19 RT-LAMP Kit
- Gyeonggi providence Promising Small Business Certificate
- FAMILY business designation certificate
- Innovative SME (Inno-Biz) certificate
- Management Innovation SME (MAIN-BIZ) certificate
- Venture business certificate
- Technical Evaluation Certificate
- Corporate Research Certificate
- Gene Research Institute Change Report
- Genetic Testing Institute Change Report
- Genetic testing accuracy assessment certificate
- Woman business ownership certificate
- CERTIFICATE EN ISO 13485
- Certification for in vitro diagnostic medical device manufacturing and quality control standards(GMP)
- In vitro Diagnostic Medical Device Manufacturing License

CE-IVD certification - DLP™Q COVID-19 RT-LAMP Kit

Gyeonggi providence Promising Small Business Certificate

FAMILY business designation certificate

Innovative SME (Inno-Biz) certificate

Management Innovation SME (MAIN-BIZ) certificate

Venture business certificate

Technical Evaluation Certificate

Corporate Research Certificate

Gene Research Institute Change Report

Genetic Testing Institute Change Report

Genetic testing accuracy assessment certificate

Woman business ownership certificate

CERTIFICATE EN ISO 13485

Certification for in vitro diagnostic medical device manufacturing and quality control standards(GMP)

In vitro Diagnostic Medical Device Manufacturing License
Licensed / Authorized Products

- More than 40 medical device manufacturing and item permits for human diagnostics
- More than 10 medical device manufacturing and item permits for animal diagnostics
- specimen transport medium (VTM)
- In vitro Diagnostic Medical Device Manufacturing License Diviral™ Transport Kit
- In vitro Diagnostic Medical Device Manufacturing License DNA Saliva Collector
- In vitro Diagnostic Medical Device Manufacturing License Gensol™C DNARNA extraction Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C APC Mutation Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C BRAF Mutation Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C BRCA Mutation Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C CDH1 Mutation Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C Chemo-resistant detection kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C Dengue 4 Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C DPC4 Mutation Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C ERBB2 Mutation Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C JAK2 Mutation Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C Malaria Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C MLH1 Mutation Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C MTHFR Mutation Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C pRB Mutation Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C PRSS1 Mutation Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C STI26 Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C TumorClean Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™C 아벨리노각막이상증(ACD) Mutation Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™Q COVID19 RT-LAMP Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™Q IFNA RT-LAMP Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™Q m.TB RT-LAMP Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™Q M.TBNTM Real Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™Q MERS RT-LAMP Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™Q PESTIS RT-LAMP Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™Q RSV RT-LAMP Kit
- In vitro Diagnostic Medical Device Manufacturing License DLP™Q STI14 Real Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License ISD™C Meningitis 11 detection kit
- In vitro Diagnostic Medical Device Manufacturing License ISD™C Pneumonia 6 detection Kit
- In vitro Diagnostic Medical Device Manufacturing License ISD™C Sepsis 20 detection Kit
- In vitro Diagnostic Medical Device Manufacturing License ISD™C STD12B Detection Kit
- In vitro Diagnostic Medical Device Manufacturing License X-Finder breast cancer miRNA Assay

specimen transport medium (VTM)

In vitro Diagnostic Medical Device Manufacturing License
Diviral™ Transport Kit
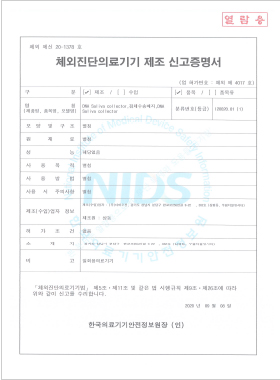
In vitro Diagnostic Medical Device Manufacturing License
DNA Saliva Collector
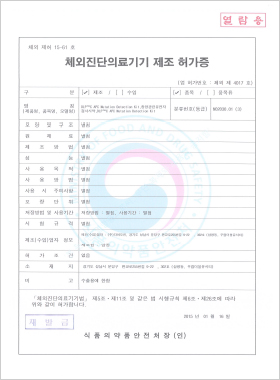
In vitro Diagnostic Medical Device Manufacturing License
Gensol™C DNARNA extraction Kit
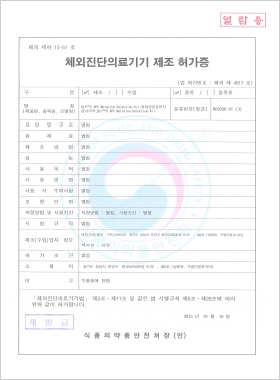
In vitro Diagnostic Medical Device Manufacturing License
DLP™C APC Mutation Detection Kit
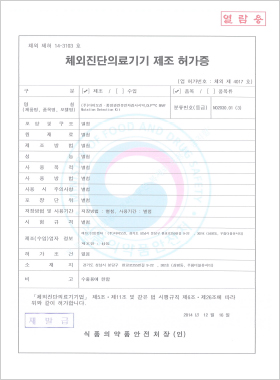
In vitro Diagnostic Medical Device Manufacturing License
DLP™C BRAF Mutation Detection Kit
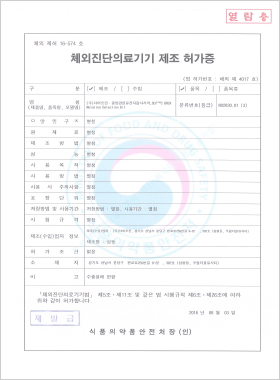
In vitro Diagnostic Medical Device Manufacturing License
DLP™C BRCA Mutation Detection Kit
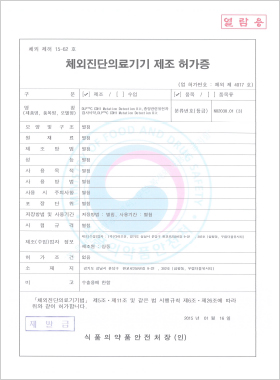
In vitro Diagnostic Medical Device Manufacturing License
DLP™C CDH1 Mutation Detection Kit
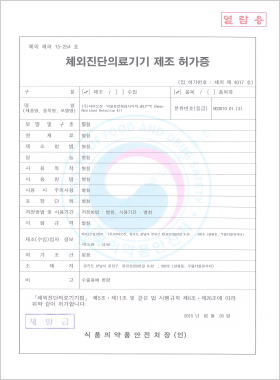
In vitro Diagnostic Medical Device Manufacturing License
DLP™C Chemo-resistant detection kit
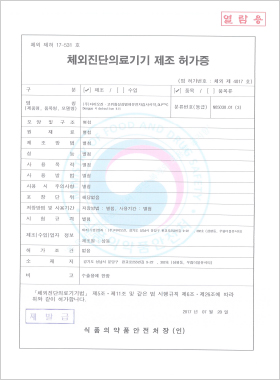
In vitro Diagnostic Medical Device Manufacturing License
DLP™C Dengue 4 Detection Kit
In vitro Diagnostic Medical Device Manufacturing License
DLP™C DPC4 Mutation Detection Kit

In vitro Diagnostic Medical Device Manufacturing License
DLP™C ERBB2 Mutation Detection Kit
In vitro Diagnostic Medical Device Manufacturing License
DLP™C JAK2 Mutation Detection Kit
In vitro Diagnostic Medical Device Manufacturing License
DLP™C Malaria Detection Kit
In vitro Diagnostic Medical Device Manufacturing License
DLP™C MLH1 Mutation Detection Kit
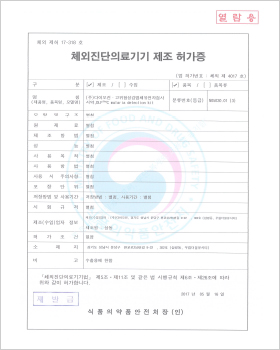
In vitro Diagnostic Medical Device Manufacturing License
DLP™C MTHFR Mutation Detection Kit
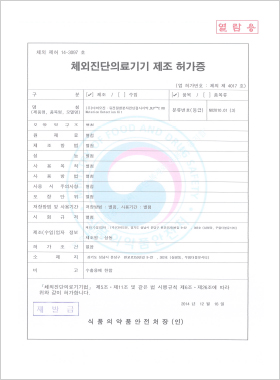
In vitro Diagnostic Medical Device Manufacturing License
DLP™C pRB Mutation Detection Kit
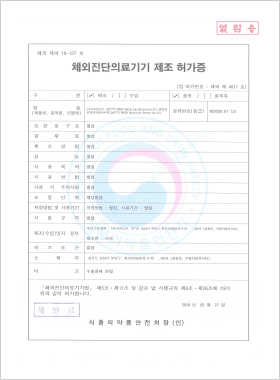
In vitro Diagnostic Medical Device Manufacturing License
DLP™C PRSS1 Mutation Detection Kit
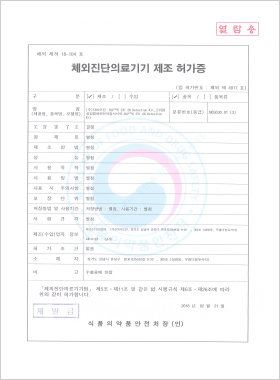
In vitro Diagnostic Medical Device Manufacturing License
DLP™C STI26 Detection Kit
In vitro Diagnostic Medical Device Manufacturing License
DLP™C TumorClean Kit
In vitro Diagnostic Medical Device Manufacturing License
DLP™C 아벨리노각막이상증
(ACD) Mutation Detection Kit
In vitro Diagnostic Medical Device Manufacturing License
DLP™Q COVID19 RT-LAMP Kit

In vitro Diagnostic Medical Device Manufacturing License
DLP™Q IFNA RT-LAMP Kit
In vitro Diagnostic Medical Device Manufacturing License
DLP™Q m.TB RT-LAMP Kit
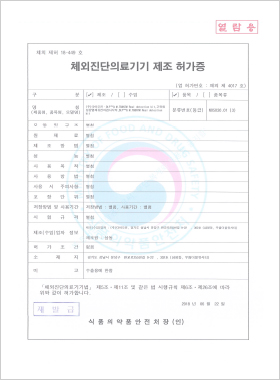
In vitro Diagnostic Medical Device Manufacturing License
DLP™Q M.TBNTM Real Detection Kit
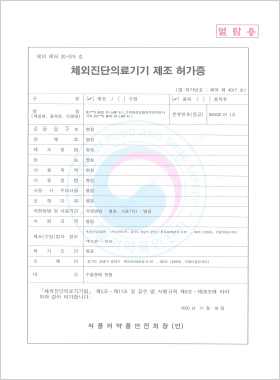
In vitro Diagnostic Medical Device Manufacturing License
DLP™Q MERS RT-LAMP Kit
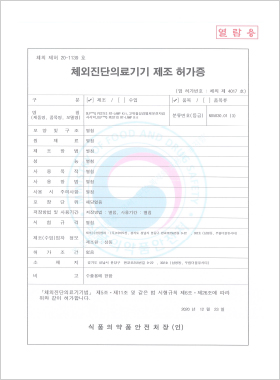
In vitro Diagnostic Medical Device Manufacturing License
DLP™Q PESTIS RT-LAMP Kit
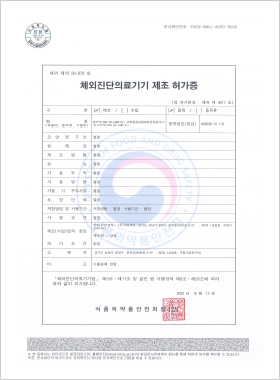
In vitro Diagnostic Medical Device Manufacturing License
DLP™Q RSV RT-LAMP Kit
In vitro Diagnostic Medical Device Manufacturing License
DLP™Q STI14 Real Detection Kit
In vitro Diagnostic Medical Device Manufacturing License
ISD™C Meningitis 11 detection kit
In vitro Diagnostic Medical Device Manufacturing License
ISD™C Pneumonia 6 detection Kit
In vitro Diagnostic Medical Device Manufacturing License
ISD™C Sepsis 20 detection Kit
In vitro Diagnostic Medical Device Manufacturing License
ISD™C STD12B Detection Kit
In vitro Diagnostic Medical Device Manufacturing License
X-Finder breast cancer miRNA Assay
